![]()
![]()
Questions? Call Us 1–773–271–0318 9am to 2pm Central Time Mon.–Fri.
3D Periodic Tables
As James Elkins says in his book How to Use Your Eyes; the standard periodic table "..serves many purposes well, but is also full of drawbacks. ...there is a big gap at the top, as if a chunk had been taken out of it. And at the bottom there are two extra strips of elements that could not be fitted onto the table."

fig.1 the standard flat form, with the blocks in different colors – showing a wide and two less wide gaps in periods at the top and the two displaced Rare Earth groups at the bottom (which belong between the left two blocks), plus a jump at the end of every period.
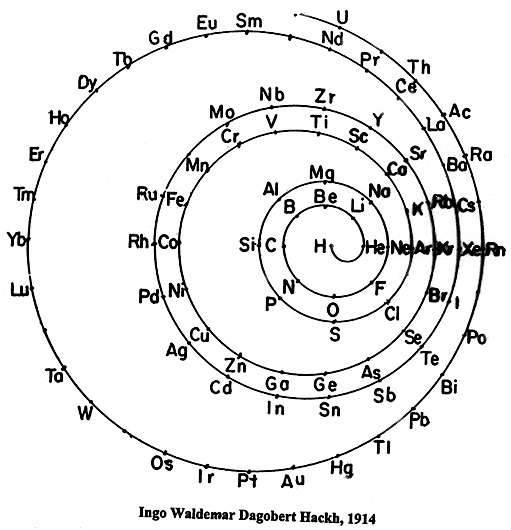
fig.2 An early spiral element arrangement by Hackh.
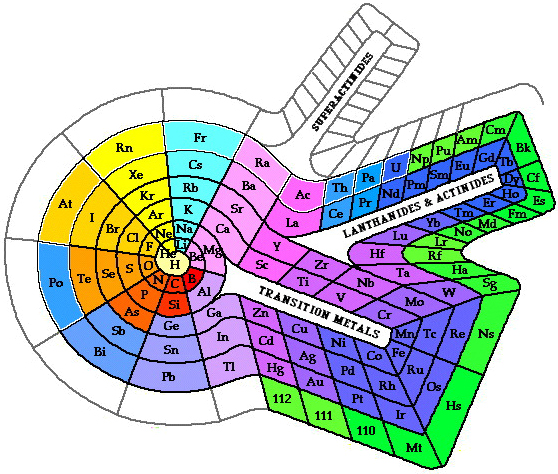
fig.3 Benfey's spiral with block extensions.
Flattening the periodic table requires placing gaps between some element blocks, jumps at the end of periods, and complete displacement of 28 elements – the Rare Earths (fig.1). The desired "Mendeleev's line" – all the elements in a row, if flat, must be a 100+ element ribbon of the successively numbered elements, or, may be achieved in three dimensions, wrapping the "line" to place element groups in adjacent positions.
Spiral arrangements are favored by periodic table developers to overcome these contradictions to the letter and the Law of periodic tables. This has been done many times previously in 2–D art as well as 3D models.
Contiguous and continuous elements arrangements of elements are possible with spiral designs, healing the incorrect and confusing gaps between blocks and joining the end of one period to the beginning of the next. Family and other relationships apparent on spiral art are hard to portray, fig.2. Also, 2–D spiral illustrations have difficulty with the inclusion of much information in the element "boxes", fig. 3.
A periodic table spiraled into 3D becomes a helix, with a printed 2–D surface curving into another dimension for new connections, figs. 4, 5. This provides an ideal plane for all the graphic, textual, and symbolic data that could be found on any table - suggesting that the form could well model the actual CHEMICAL ELEMENT SYSTEM.
While three–dimensional helical tabular periodic tables may appear novel, they are historically correct. As a matter of fact, the very first true periodic table was such a 3D spiral, a simple helix of the available elements at the time by Alexandre–Emile de Chancourtois in 1862, fig. 6. Over 10 years later, Mendeleev developed the Periodic Law, which has been the guiding force behind all periodic tables since.

fig.4 20th Century Inventors of 3D Periodic Element Arrangements –
Courtines, Gamov, Denker, and Alexander

fig.5 The Alexander Arrangement of Elements.

fig.6 Alexandre Emile Beguyer de Chancourtois invented the periodic table, in 3D, in 1862, about 7 years prior to the announcement of Meyer’s and Mendeleev’s flat periodic tables.
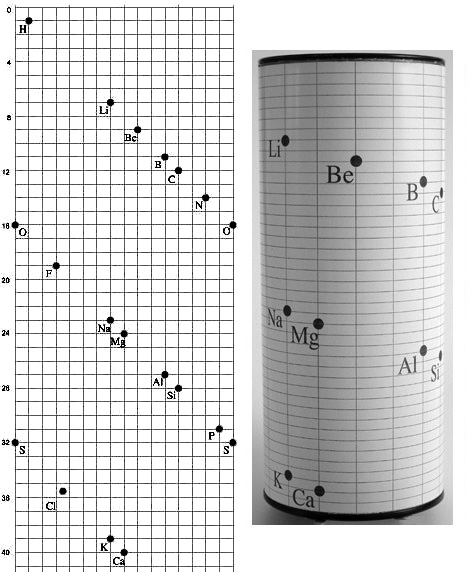
fig.7 de Chancourtois' Vis Tellurique.
AlexanderDESIGN
4851 N. Washtenaw Ave., Chicago, IL 60625
last update 9/20/16