Putting It Together
Kids – especially boys – love to take things apart.
Most Moms attribute this to the basic destructiveness of the male animal, but that is not usually the case. It is to set up a situation where,having all the parts conveniently still at hand, one can put something together. The dismembering is the preparation for the creative act, while not completely original, (as one can remember that it was a functioning something once before,) at least a challenge to make something functional, and blessedly without need for instructions.
Being told what to do, for boys, is a pain. It is probably linked to the Y chromosome, as I understand that lost male drivers worldwide have similar difficulties asking for directions.
That being said, manually building a model, especially of the Periodic Table of the Elements will certainly appeal to a larger number of students than observing the wallpaper style flat table.
The DeskTopper Alexander Arrangement of Elements provides the requirement to take something apart, and then to put it together again.Students approaching their very first standard Periodic Table Lesson (dreaded equally by student and teacher) have the opportunity to incorporate their prior knowledge – without real need for any – in an activity that transitions them to the understanding and acceptance of the flat periodic table that is at the core of the study of Chemistry.
The segments of the DeskTopper are pre–cut on a single sheet, no instructions, blades, or scissors needed for removal. There is an instruction sheet, to be sure, for those willing to read them, and, of course, a series of assembly drawings for the less bold – not to mention photos of the final product.
(Interestingly, one section, formed into a tube, is virtually the same as the very first periodic table, that of Alexandre–Emile Beguyer de Chancourtois, published 10 years before Dmitri Mendeleev started us on the flat periodic table thing.


This tube makes up the s– and p–blocks, and the students learn about blocks visually, intellectually, and tactilly ...if there is such a word.)
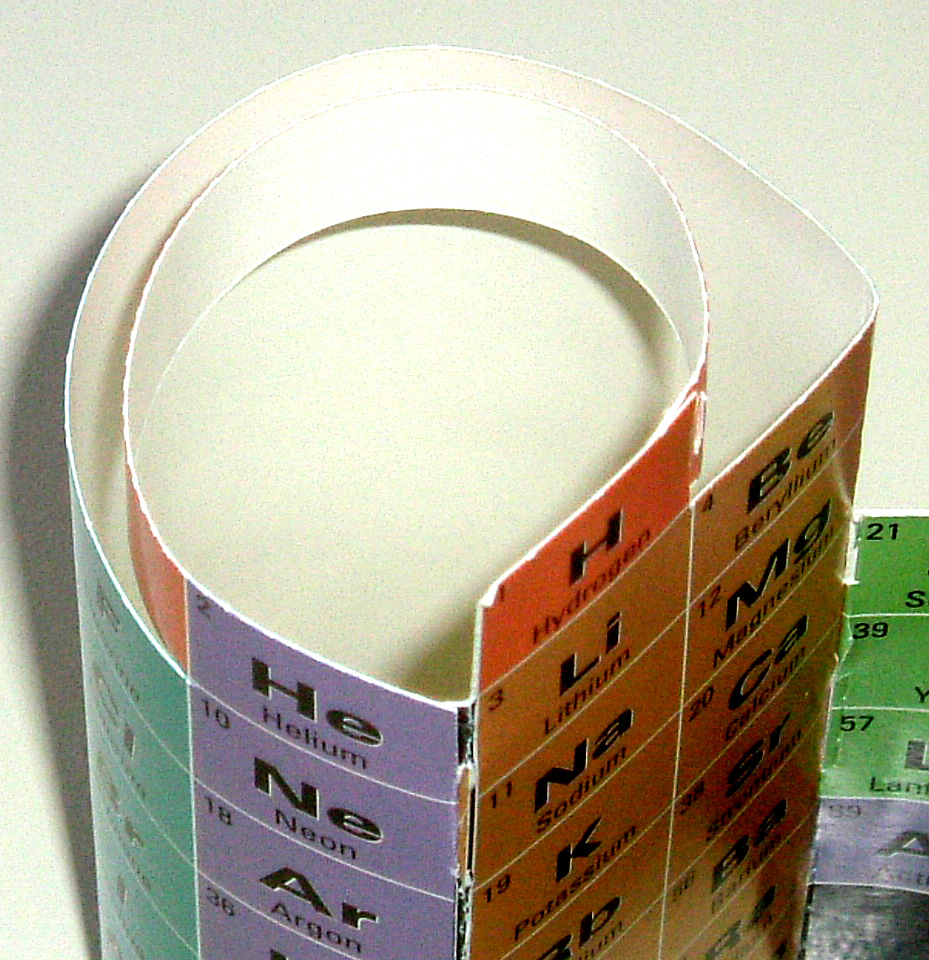
The two elements of the first period are connected with an extended Hydrogen box (now a strip) lending special status to this most basic atom, the original element of the universe and that with which the Sun warms and illuminates us.
The element atomic number jump, from the end of one period to the beginning of the next has changed to contact, between 2 to 3, 10 to 11, and 18 to 19.
The students, with glue, staples, or tape, loop and make teardrop shapes out of the two remaining segments – d–block and f–block – which can be studied independently. (Talk about Hands–On!) With both of these integrated, not only are all of the Mendeleev imposed awkward gaps of the flat table closed, but the Seaborg–orphaned Rare Earths are welcomed home as well.
“Fear of Periodic Table” has thus been defused, and, with a historical assembly sequence, the logic of the Periodic Law becomes obvious; “...if all the elements be arranged in order of their atomic weights, a periodic repetition of properties is obtained...”. The elements are understood to be continuous and contiguous in atomic number as well as properties, leading to an uncritical approach, in the next lesson, to the flat–for–convenience–sake periodic table that students will use from now on.
Using the Alexander Arrangement of Elements in the DeskTopper form with this lesson, students have, in a fun activity, interacted with the periodic table more than ever possible before, held the individual blocks in their hands, learned the how and why of the positions and connections of the blocks, and have a souvenir of this lesson that some might keep forever to remember a GREAT lesson/teacher.